C-mAIn@annauniv.edu
044-22359938
Pollution Mitigation
"Exploration of Oxide Surfaces for Environmental Pollution Reduction"
Anthropogenic emission of Nitrous oxide (NOx) in the atmosphere is harmful to human beings
as well as to
the environment by causing air pollution, acid rain, and greenhouse effect. Reducing the
polluting NOx
contents into harmless N2 and O2 gases has been of immense research interest recently. One
of the most
promising approaches is the photocatalytic reduction. Surfaces made of transition-metal
oxides such as
TiO2, SnO2 and ZnO etc are able to carry out these photocatalytic redox reactions by
incidence of solar
Among them TiO2 is considered as the most promising semiconductor photocatalyst due to its
high stability,
non-toxicity and cost-effectiveness and has enormous applications in many fields like
renewable energy,
environmental protection, industries etc. TiO2 surfaces are also helpful in the
photocatalytic reduction
of NOx gas molecules. However, pure TiO2 has a wide bandgap (> 3eV) which makes it active in
the UV-region
of solar spectrum, whereas solar irradiance is maximum in the visible region (1.6 to 3.2
eV).
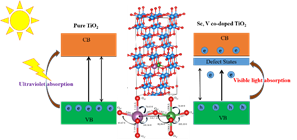
Therefore, to reduce the band-gap of TiO2 and make it more photocatalytically active in the
visible region
of solar spectrum, we have been engineering its band-gap by using Density functional theory.
We have
analyzed the effects of transition-metal dopants on the band-gap of TiO2, and shown that Nb
and W doping
can make TiO2 a active photocatalyst in the visible-light.
The Scanning Tunneling Microscope (STM) usually provides illustrations of the surface
morphology, surface
defects and conformation of molecules and is a great tool in characterizing the surfaces of
materials.
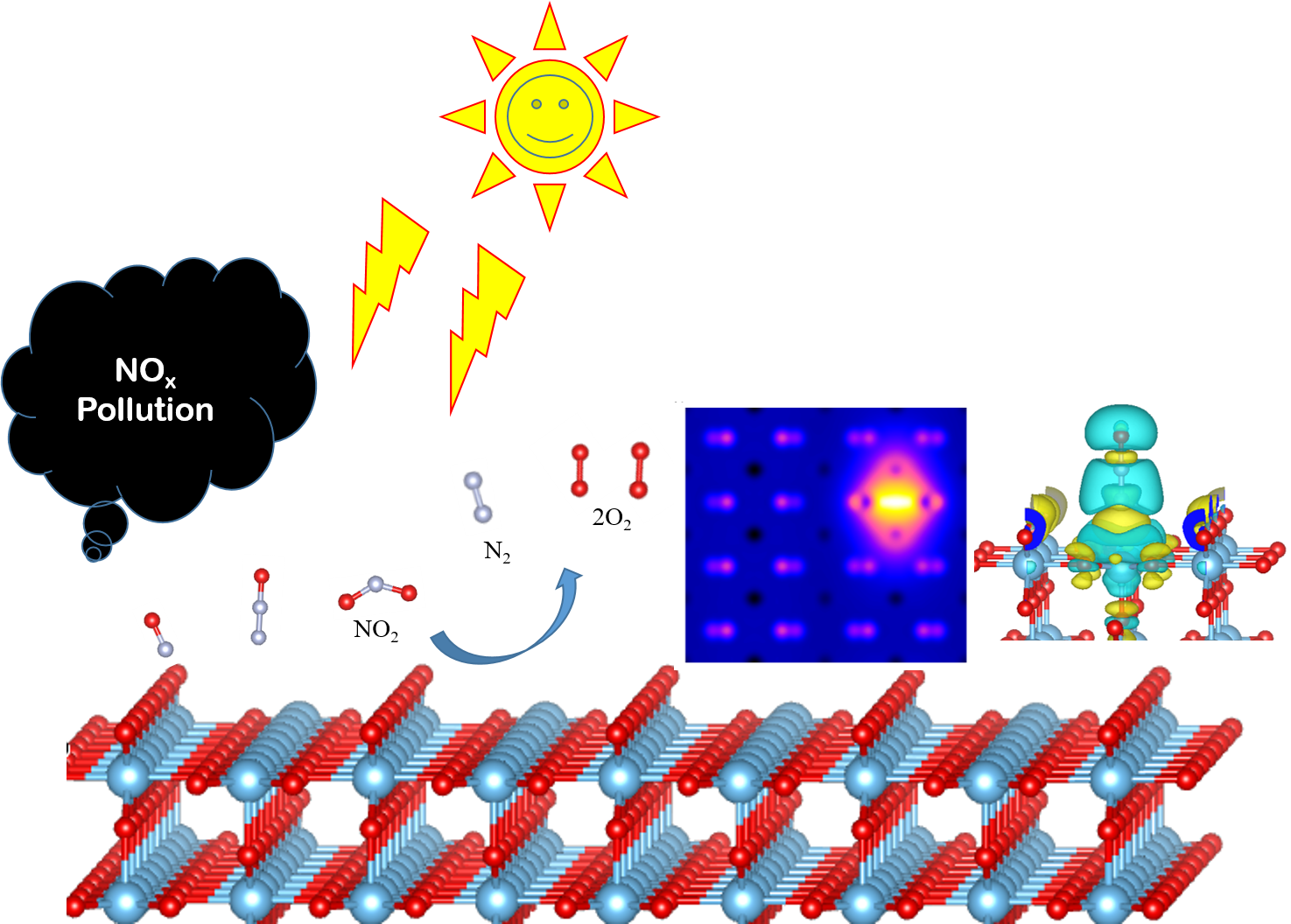
Using DFT we have been simulating the STM images of the pure and NOx adsorbed TiO2 surfaces
under various
configurations and conformations of the adsorbent molecules. Our work throws more light on
the bonding nature
of the TiO2 surface, best suitable NOx conformation for more adsorption, and interaction
mechanism between the
TiO2 surface and the adsorbents
We have been also modelling heterostructures of various transition-metal oxides, so that we
can identify
a new photacatalyst with enhanced capacity for NOx abatement. In addition, clusters of
metal-oxides are
modelled on top of TiO2 surface for seeking better photocatalysts.
Quick Links
Get In Touch
Sir CV Raman Block , Anna University.
C-mAIn@annauniv.edu
044-22359938
© C-mAIn. All Rights Reserved. Designed by HTML Codex. Maintained by Scholars of C-mAIn.