
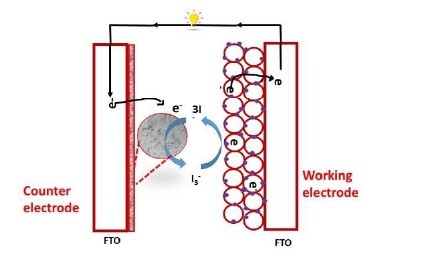
Dye-sensitized solar cell (DSSC) as a promising renewable energy source over other photovoltaic devices for its simple manufacturing process, flexibility, high energy conversion and low production cost. DSSCs consists of an anode coated with conducting oxide, platinum as counter electrode and electrolyte. During the DSSCs performance, the CE catalyze the electrolyte redox couple (I¯/I¯3) and completes the electrical circuit. In the performance of DSSC, the redox electrolyte couple (I¯/I¯3) reduces the photo induced dye molecule and oxidize I¯3 species through diffusion from dye molecule to CE and thereby regenerating I¯. Platinum is widely used catalyst in spite of its high cost for CE in DSSC, however the major drawback of platinum is its decomposition to PtI4 by (I¯/I¯3) redox couple, thus resulting in the reduced efficiency of the cell and its stability. Therefore, it is necessary to replace an alternate CE with other materials for an enhanced efficiency, durability and fabrication cost. In recent years, researchers have focused on transition metal nitrides and metal oxides; as it exhibits noble metals electronic configuration, they have good electro analytic activity, high conductivity and significant chemical corrosion resistance. Therefore, metal nitrides have similar structure as noble metals. Among these counter electrode materials vanadium nitrides (VN) is highly prominent with the advantage of corrosion resistance, low cost and enhanced catalytic activity with good chemical stability towards I¯/I3¯ redox reaction. Vanadium nitride possesses slightly increase charge transfer resistance at electrode/electrolyte interface due to the formation of vanadium oxide on VN lattice, to overcome the further formation of oxide on VN surface by intercalated graphene to the VN lattice. It also enhances the electronic conductivity of the VN-Graphene (VNGp) matrix. As a result, VNGp acts as a better electrocatalyst for the I3¯/I¯ redox reaction. The VNGp hybrid counter electrode displayed an adsorption-controlled process towards the (I¯/I3¯) redox couple at CE/electrolyte interface and the value was calculated to be 3.16 x10–9 mol/s by varying the scan rate. The VNGp hybrid counter shows a maximum fill factor of 66.28 with high efficacy of 8.15% under the illumination of 100 mW/cm2 as compared to the Pt CE for DSSC.
The Centre for Energy Storage Technologies [CEST] is one of the leading research centres on all aspects of electrical energy storage in India. The CEST is primarily emphasis on the Development of electrochemical energy storage devices with high power density including battery, supercapacitors and Power Dense Devices. The CEST Centre was formed in 2022 to bring together the campus-wide expertise in energy storage, foster collaboration, and provide a focal point for research and education activities.